Answer: c. so that you use the correct energy states
Step-by-step explanation:
Enthalpy is the energy release or energy produced when a chemical reaction is carried out.
Enthalpy change is calculated by substracting the energy of reactants from the energy of products.
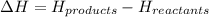
As the energies are different for different states and thus the correct stale symbols in your balanced chemical equation must be given so that we use the correct energy values.