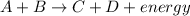Answer: The reaction is endothermic.
Step-by-step explanation:
Endothermic reaction : It is a type of chemical reaction where the energy is absorbed from the surrounding. In the endothermic reaction, the reactant have less energy than the energy of products
In endothermic reaction, enthalpy terms is located on the reactant side.
Example:
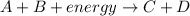
Exothermic reaction : It is a type of chemical reaction where the energy is released into the surrounding. In the exothermic reaction, the energy of reactants are more than the energy of products
In exothermic reaction, enthalpy terms is located on the product side.
Example: