Answer:
The volume of 9.0 M copper (II) sulfate stock solution needed to prepare 3.0 L of a 5.0 M solution is 1.667 L
Step-by-step explanation:
Dilution is a process by which the concentration of a solute in solution is reduced by adding more solvent.
In other words, dilution is the procedure followed to prepare a less concentrated solution from a more concentrated one, and it simply consists of adding more solvent.
In a dilution the amount of solute does not vary. What varies in a dilution is the volume of the solvent: as more solvent is added, the concentration of the solute decreases, as the volume (and weight) of the solution increases.
The equation used in this case is:
Ci * Vi = Cf * Vf
where
- Ci: initial concentration
- Vi: initial volume
- Cf: final concentration
- Vf: final volume
In this case:
- Ci: 9 M
- Vi: ?
- Cf: 5 M
- Vf: 3 L
Replacing:
9 M*Vi= 5 M* 3 L
Solving:
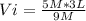
Vi= 1.667 L
The volume of 9.0 M copper (II) sulfate stock solution needed to prepare 3.0 L of a 5.0 M solution is 1.667 L