Answer:
XH2 = 0.5185; XN2 = 0.3782; XAr = 0.103
Step-by-step explanation:
Let's say the mole fraction is Xi, i is individual gas, and Pi is partial pressure.
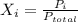
Ptotal is all the pressure added together, which is:
449.0torrH2 + 327.5torrN2 + 89.5torrAr = 866.0torr
From this point, it's pretty simple, just plug the values into the equation (watch out sig figs):
XH2 = 449.0torr/866.0torr = 0.5185
XN2 = 327.5torr/866.0torr = 0.3782
XAr = 89.5torr/866.0torr = 0.103