Answer:
800.3 Kelvin
Step-by-step explanation:
127°C = 400.15 Kelvin (because 127 + 273.15)
Charles Law:
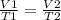 Thus,
Thus,
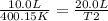
Then, work out the math algebraically.
T2 = 20.0 / (10.0/400.15)
= 20.0 / 0.02499...
= 800.3 Kelvin
This is assuming the pressure remains constant.