Answer:
We can not swim in 1.00 × 10²⁷ molecules of water
Step-by-step explanation:
The given number of molecules of water = 1.00 × 10²⁷ molecules
The Avogadro's number,
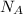 , gives the number of molecules in one mole of a substance
, gives the number of molecules in one mole of a substance
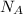 ≈ 6.0221409 × 10²³ molecules/mol
≈ 6.0221409 × 10²³ molecules/mol
Therefore
Therefore, we have;
The number of moles of water present in 1.00 × 10²⁷ molecules, n = (The number of molecules of water) ÷
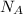
∴ n = (1.00 × 10²⁷ molecules)/(6.0221409 × 10²³ molecules/mol) = 1,660.53902857 moles
The mass of one mole of water = The molar mass of water = 18.01528 g/mol
The mass, 'm', of water in 1,660.53902857 moles of water is given as follows;
Mass = (The number of moles of the substance) × (The molar mass of the substance)
∴ The mass of the water in the given quantity of water, m = 1,660.53902857 moles × 18.01528 g/mol ≈ 29.9150756 kg.
The density pf water, ρ = 997 kg/m³
Volume = Mass/Density
∴ The volume of the water present in the given quantity of water, v = 29.9150756 kg/(997 kg/m³) ≈ 30.0050909 liters
The volume of the water present in 1.00 × 10²⁷ molecules of water ≈ 30.0 liters
The average volume of a human body = 62 liters
Therefore, we can not swim in the given quantity of 1.00 × 10²⁷ molecules = 30.0 liters water