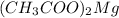Answer: Molecular formula of copper (II) bromide is
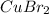
Molecular formula of aluminium (III) nitrate is
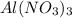
Molecular formula of calcium (II) phosphate is
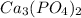
Molecular formula of iron (III) sulphide is
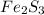
Molecular formula of mercury (II) chloride is
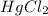
Molecular formula of magnesium (II) acetate is
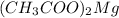
Step-by-step explanation:
The name of the ionic compounds is written by writing the name of the cation first followed by its oxidation state in round brackets and then the name of the anion is written without any suffix. Thus the cation is written first followed by the oxidation state and then the anion.
For formation of a neutral ionic compound, the charges on cation and anion must be balanced. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral compound.
Molecular formula of copper (II) bromide is
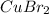
Molecular formula of aluminium (III) nitrate is
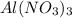
Molecular formula of calcium (II) phosphate is
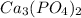
Molecular formula of iron (III) sulphide is
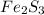
Molecular formula of mercury (II) chloride is
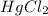
Molecular formula of magnesium (II) acetate is