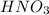Answer: e. none of these
Explanation:
Mass percent is the ratio of mass of solute dissolved to the mass of solution in terms of percentage.
Thus 71.0%
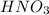 means 71.0 g of
means 71.0 g of
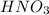 is present in 100 g of solution
is present in 100 g of solution
To calculate the number of moles, we use the equation:
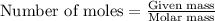
given mass of
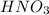 = 71.0 g
= 71.0 g
Molar mass of
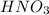 = 63.01 g/mol
= 63.01 g/mol
Putting in the values we get:
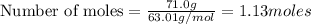
Thus there are 1.13 moles of