Answer:
The temperature of the gas will be 2630 K
Step-by-step explanation:
Charles's Law consists of the relationship that exists between the volume and the temperature of a certain quantity of ideal gas, which is maintained at a constant pressure, by means of a constant of proportionality that is applied directly. For a given sum of gas at a constant pressure, as the temperature increases, the volume of the gas increases and as the temperature decreases, the volume of the gas decreases because the temperature is directly related to the energy of the movement of the gas molecules. .
In summary, Charles's law is a law that says that when the amount of gas and pressure is kept constant, the ratio that exists between the volume and the temperature will always have the same value:
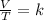
Assuming that you have a certain volume of gas V1 that is at a temperature T1 at the beginning of the experiment, by varying the volume of gas to a new value V2, then the temperature will change to T2, and it will be fulfilled:
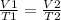
In this case:
- V1= 130 L
- T1= 3750 C=4023 K (being 0 C=273 K)
- V2= 85 L
- T2= ?
Replacing:
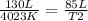
Solving:
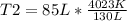
T= 2630 K
The temperature of the gas will be 2630 K