Answer:
T₂ = 800 K
Step-by-step explanation:
Given that,
Initial temperature of the gas, T₁ = 127°C = 400 K
Initial volume, V₁ = 10 L
Final volume, V₂ = 20 L
We need to find the new temperature.
The volume of a gas is directly proportional to its Kelvin temperature.
Let T₂ is the new temperature. So,
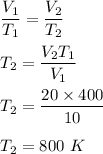
So, the new temperature of the gas is 800 K.