1. Only the fourth equation
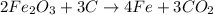 is unbalanced.
is unbalanced.
2. covalent, different, 1, 4, 1, 8, 2, and stable.
How did we arrive at these assertions?
1. To determine if the given chemical equations are balanced or unbalanced, we need to check whether the number of atoms of each element on the reactant side is equal to the number of atoms of the same element on the product side.
1.
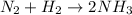
- Nitrogen (N): 2 on both sides
- Hydrogen (H): 2 on both sides
- Balanced: Yes
2.
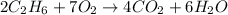
- Carbon (C): 4 on both sides
- Hydrogen (H): 12 on both sides
- Oxygen (O): 14 on both sides
- Balanced: Yes
3.

- Zinc (Zn): 4 on both sides
- Sulfur (S): 4 on both sides
- Oxygen (O): 14 on both sides
- Balanced: Yes
4.
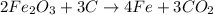
- Iron (Fe): 4 on both sides
- Carbon (C): 3 on both sides
- Oxygen (O): 10 on both sides
- Unbalanced: No
5.

- Carbon (C): 3 on both sides
- Hydrogen (H): 8 on both sides
- Oxygen (O): 10 on both sides
- Balanced: Yes
So, only the fourth equation
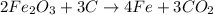 is unbalanced.
is unbalanced.
2. The bonds in methane are covalent because hydrogen and carbon have different electronegativity. Carbon bonds with four hydrogen atoms because hydrogen has 1 valence electron(s) and carbon has 4 valence electrons; so carbon shares 1 valence electron with each of the four hydrogen atoms to satisfy the necessary 8 valence electrons for carbon and 2 for hydrogen to become stable.