Final Answer:
The electric force on a proton 2.3 femtometers (fm) from the surface of the nucleus, treating the nucleus as a point charge, can be calculated using Coulomb's law and is
![\[F = (k \cdot |q_1 \cdot q_2|)/(r^2)\]](https://img.qammunity.org/2024/formulas/mathematics/high-school/t5udgst2qkzldkximqy78180qggcxicmj2.png) , where
, where
 is the force,
is the force,
 is Coulomb's constant,
is Coulomb's constant,
 are charges (proton's charge and the nucleus's charge, respectively), and
are charges (proton's charge and the nucleus's charge, respectively), and
 is the separation distance.
is the separation distance.
Explanation:
Utilizing Coulomb's law
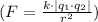 , where
, where
 is Coulomb's constant
is Coulomb's constant
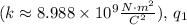 represents the charge of the proton
represents the charge of the proton
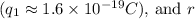 is the distance from the proton to the nucleus's surface (given as
is the distance from the proton to the nucleus's surface (given as
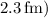 . Assuming the nucleus as a point charge
. Assuming the nucleus as a point charge
 , the force can be calculated.
, the force can be calculated.
Substituting the known values into Coulomb's law yields:
![\[F = ((8.988 * 10^9 (N \cdot m^2)/(C^2)) \cdot |(1.6 * 10^(-19) C) \cdot q_2|)/((2.3 * 10^(-15) m)^2)\]](https://img.qammunity.org/2024/formulas/mathematics/high-school/9dj1s85wkhsjm6ouj3olvxoicq7oifizi0.png)
The value of
 (charge of the nucleus) needs to be known or provided to precisely calculate the electric force acting on the proton at a distance of 2.3 femtometers from the nucleus's surface. Coulomb's law determines the force between charged particles, and in this case, the force on the proton depends on the charge of the nucleus, which is assumed to be concentrated at the nucleus's center. Therefore, knowledge of the nucleus's charge is crucial for an accurate calculation of the electric force acting on the proton at the specified distance.
(charge of the nucleus) needs to be known or provided to precisely calculate the electric force acting on the proton at a distance of 2.3 femtometers from the nucleus's surface. Coulomb's law determines the force between charged particles, and in this case, the force on the proton depends on the charge of the nucleus, which is assumed to be concentrated at the nucleus's center. Therefore, knowledge of the nucleus's charge is crucial for an accurate calculation of the electric force acting on the proton at the specified distance.