Answer:
Approximately
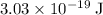 , which corresponds to a wavelength of approximately
, which corresponds to a wavelength of approximately
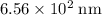 in vacuum.
in vacuum.
Step-by-step explanation:
When the electron of a hydrogen atom transits from energy level
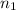 to
to
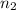 , the energy change would be:
, the energy change would be:
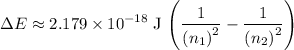 .
.
For the transition from
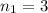 to
to
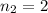 :
:
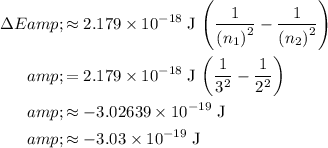 .
.
The value of
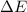 is negative because energy is released during this transition.
is negative because energy is released during this transition.
Look up Planck's Constant (for finding frequency from energy) and the speed of light in vacuum (for finding wavelength from frequency.)
- Planck's Constant:
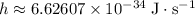 .
. - Speed of light in vacuum:
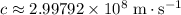 .
.
Calculate the frequency
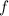 of photons from this transition using the Planck-Einstein relation:
of photons from this transition using the Planck-Einstein relation:
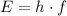 .
.
Therefore:
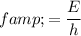 .
.
Calculate the wavelength
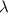 of these photos in vacuum:
of these photos in vacuum:
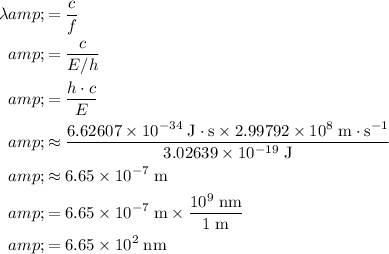 .
.