Answer:
NaOH is the limiting reactant.
204.9 g of sodium phosphate are formed.
51.94 g of excess reactant will remain.
Step-by-step explanation:
The reaction that takes place is:
- H₃PO₄ + 3NaOH → Na₃PO₄ + 3H₂O
First we convert the mass of both reactants to moles, using their respective molar masses:
- H₃PO₄ ⇒ 175 g ÷ 98 g/mol = 1.78 mol
- NaOH ⇒ 150 g ÷ 40 g/mol = 3.75 mol
1.78 moles of H₃PO₄ would react completely with (1.78 * 3) 5.34 moles of NaOH. There are not as many NaOH moles so NaOH is the limiting reactant.
--
We calculate the produced moles of Na₃PO₄ using the limiting reactant:
- 3.75 mol NaOH *
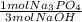 = 1.25 mol Na₃PO₄
= 1.25 mol Na₃PO₄
Then we convert moles into grams:
- 1.25 mol Na₃PO₄ * 163.94 g/mol = 204.9 g
--
We calculate how many H₃PO₄ moles would react with 3.75 NaOH moles:
- 3.75 mol NaOH *
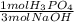 = 1.25 mol H₃PO₄
= 1.25 mol H₃PO₄
We substract that amount from the original amount:
- 1.78 - 1.25 = 0.53 mol H₃PO₄
Finally we convert those remaining moles to grams:
- 0.53 mol H₃PO₄ * 98 g/mol = 51.94 g