Answer:
2.14 g.
Step-by-step explanation:
Hello!
In this case, since the mole ratio between nitrogen and oxygen is 2:5 respectively, we realize we first need the moles of nitrogen given it's atomic mass (14.01 g/mol):
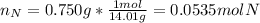
Now, we calculate the moles of oxygen:
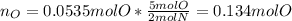
Then, we compute the moles of oxygen given its atomic mass (16.00 g/mol):
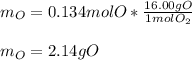
Best regards!