Answer:
Lead, Pb.
Step-by-step explanation:
Hello!
In this case, since the molar mass of the compound is 293.8 g/mol, and the molecular formula is NaXO₄, we infer that the molar mass is computed via:
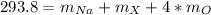
Thus, we can solve for the atomic mass of X as shown below:
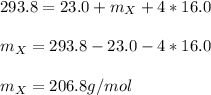
Thus, the element whose atomic mass is about 206.8 g/mol is lead (207.2 g/mol) even when it is not properly a transition metal.
Best regards!