Answer:
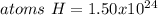
Step-by-step explanation:
Hello!
In this case, since the molecular formula of methane is CH₄, can compute the moles of methane in 10 g first:
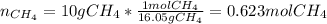
Now, since 1 mol of methane contains 4 mol of hydrogen atoms, and 1 mol of any atom contains 6.022x10²³ atoms via the Avogadro's number, we obtain the following number atoms of hydrogen:
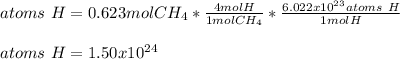
Best regards!