Answer:
(a) 313.4 grams
(b) 35.1 years
Explanation:
Strontium 90 is a radioactive isotope that decays according to the given equation:
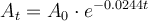
where:
 is the initial amount present.
is the initial amount present.
 is the amount present after t years.
is the amount present after t years.
Given that that the initial amount of strontium 90 is 400 grams, we can substitute A₀= 400 into the given equation:
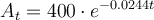

Part (a)
To determine how much strontium 90 will be left after 10 years, we can substitute t = 10 into the given equation:
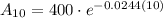
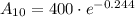
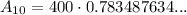
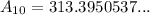
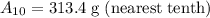
Therefore, there will be 313.4 grams of strontium 90 left after 10 years.

Part (b)
To determine when 170 grams of strontium 90 will be left, we can substitute
 into the equation and solve for t:
into the equation and solve for t:
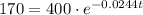
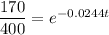
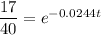
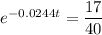
Take natural logs of both sides of the equation:
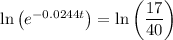
Apply the log power rule to the left side of the equation:
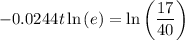
As ln(e) = 1, then:
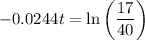
Divide both sides by -0.0244:
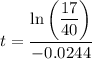
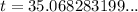
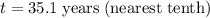
Therefore, there will be 170 grams of strontium 90 left after 35.1 years.