The following table shows the concentrations of [
 ], [F], [HF], [OH], and [
], [F], [HF], [OH], and [
 ] in a saturated solution of
] in a saturated solution of
 , as determined using the Solver function in Excel:
, as determined using the Solver function in Excel:
Variable Concentration
[
 ] 9.33 x
] 9.33 x
 M
M
[F] 1.87 x
 M
M
[HF] 1.61 x
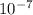 M
M
[OH] 1.61 x
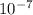 M
M
[
 ] 6.23 x
] 6.23 x
 M
M
To solve this problem using Solver, we can first set up a spreadsheet with the following columns:
Variable: This column will contain the names of the variables we want to solve for ([
 ], [F], [HF], [OH], and [
], [F], [HF], [OH], and [
 ]).
]).
Initial value: This column will contain our initial guesses for the values of the variables.
Constraint: This column will contain the constraints that the variables must satisfy.
For example, we know that the Ksp of
 is 2.63 x
is 2.63 x
 , so we can set up the following constraint:
, so we can set up the following constraint:
![[Sr_2^+] * [F]^2](https://img.qammunity.org/2024/formulas/chemistry/high-school/djcse2ai3xuzhcvl3fd5fn75debrkooprt.png) = 2.63 x
= 2.63 x

We also know that the Ka of HF is 6.8 x
 , so we can set up the following constraint:
, so we can set up the following constraint:
[HF] / [F] = 6.8 x

Finally, we know that the pH of a neutral solution is 7, so we can set up the following constraint:
![[H^+] * [OH^-] = 1 * 10^(-14)](https://img.qammunity.org/2024/formulas/chemistry/high-school/c176fm7gvaqzwamfh90sfw9oc326vpelb9.png)
Once we have set up our spreadsheet, we can open the Solver tool and enter the following information:
Set Objective: Set the objective cell to the cell containing the constraint that you want to minimize. In this case, we want to minimize the difference between the calculated value of
![[H^+] * [OH^-]](https://img.qammunity.org/2024/formulas/chemistry/high-school/tso6qqcxfu807j3uly5dh8hdm5o5b4kxif.png) and
and

By Changing Variable Cells: Select the cells containing the initial guesses for the values of the variables. In this case, we want to solve for [
 ], [F], [HF], [OH], and [
], [F], [HF], [OH], and [
 ].
].
Subject to the Constraints: Enter the constraints that you want the Solver to satisfy. In this case, we have three constraints:
![[Sr_2^+] * [F]^2 = 2.63 * 10^(-9)](https://img.qammunity.org/2024/formulas/chemistry/high-school/6a2ke6knsbvbzn01xjo67wbapb7d2w8i05.png)
![([HF])/([F]) = 6.8 * 10^(-4)](https://img.qammunity.org/2024/formulas/chemistry/high-school/sepefv8z7r72d80hscn2ckvqn3gsdaw08m.png)
![[H^+] *[OH^-] = 1 * 10^(-14)](https://img.qammunity.org/2024/formulas/chemistry/high-school/jcg8eb3thmmcgky25c4uzzo6rzh6u65hug.png)
Once you have entered all of the necessary information, click the "Solve" button. The Solver will then calculate the values of the variables that satisfy all of the constraints.
Question:-
Use the Solver function in Excel to determine the concentrations of [
 ], [F], [HF], [OH], and [
], [F], [HF], [OH], and [
 ] for a saturated solution of SrF2, given the pKsp of SrF2 (8.58) and the pKa of HF (3.17).
] for a saturated solution of SrF2, given the pKsp of SrF2 (8.58) and the pKa of HF (3.17).