Decreasing the concentration of
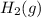 will decrease the production of
will decrease the production of
 (g). Option C is the right choice.
(g). Option C is the right choice.
The reaction produces fewer moles of gas on the product side than the reactant side. Therefore, increasing the pressure will favor the forward reaction (fewer gas molecules) and increase
 production, eliminating (B) as an option.
production, eliminating (B) as an option.
Decreasing the temperature will favor the exothermic reaction (forward reaction) and increase
 production, eliminating (D) as an option.
production, eliminating (D) as an option.
Increasing the concentration of
 will shift the equilibrium to the right according to Le Chatelier's principle, increasing
will shift the equilibrium to the right according to Le Chatelier's principle, increasing
 production, eliminating (A) as an option.
production, eliminating (A) as an option.
Therefore, the only stress that will decrease
 production is (C) decreasing the concentration of
production is (C) decreasing the concentration of
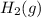 , as it shifts the equilibrium to the left, favoring the reactants.
, as it shifts the equilibrium to the left, favoring the reactants.
Option C is the right choice.