Answer:
41.88375 g Fe
Step-by-step explanation:
Using stoichiometry, we can do this problem. First, we put our given. Then we create an equation using the molar mass of Fe (found on the periodic table, which was 55.845 g Fe), and the resulting equation is:
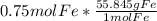
The mol Fe's cancel, and leave us with:
41.88375 g Fe
Good luck.