Answer:
1. Some combination of ions form a solid precipitate because it is not favorable for the ions to become solvated (dissolved). Large and lowly charged ions tend to form precipitates, especially metals such as lead, barium, and silver.
2.
a. Pb(NO3)2 + 2KI -> 2KNO3 + PbI2
b. PbI2 is a precipitate because no other combinations of cations and anions will make an insoluble compound. KI, KNO3, and Pb(NO3)2 are all soluble.
c.
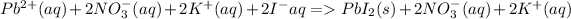
is the ionic equation. Spectator ions are NO3- and K+
d.
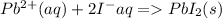 is the net ionic equation
is the net ionic equation
ask questions in comments if you have any