Answer:
26.9 L.
Step-by-step explanation:
Hello there!
In this case, since the ideal gas equation is able to provide us the volume of neon in 1.2 moles by considering the STP conditions (1.00 atm and 273.15 K) via its mathematical definition:

We first need to solve for V in the aforementioned equation:
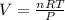
So we plug in to obtain:

Best regards!