Answer: 2.23 moles
Step-by-step explanation:
I am going to say, first, that the formula for butane is not
 , but that it is actually
, but that it is actually
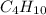 .
.
Using the formula for butane, we can see that the balanced equation for the combustion of butane is
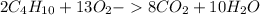 .
.
We need to remember that, for an ideal gas at STP, the molar volume is 22.4 L/mol, thus we have
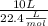 =0.446 moles of butane. We can see that the moles of H2O produced is 5 times the moles of butane used, thus, there are 0.446 * 5 or about 2.23 mol of water formed (rounding errors are bound to occur).
=0.446 moles of butane. We can see that the moles of H2O produced is 5 times the moles of butane used, thus, there are 0.446 * 5 or about 2.23 mol of water formed (rounding errors are bound to occur).