Answer:
82.9% Is the percent yield
Step-by-step explanation:
First, we need to balance the equation.
Ca(CO3) + 2HCl → H2O + CO2 + CaCl2
Since the problem tells you that the excess reactant is Ca(CO3), we just need to Stoichiometry to solve for the theoretical yield.
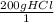 x
x
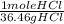 x
x
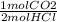 x
x
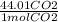 =120.707 g (theoretical yield)
=120.707 g (theoretical yield)
Take your actual yield of 100g, divide that by 120.707, then multiply that decimical by 100.