1. Empirical Formula:

2. Molecular Formula:

To determine the empirical formula and molecular formula, we can use the given percentages and molar mass.
Let's assume we have 100 g of the compound, and then we can determine the number of moles of each element.
1. **Calculate the moles of each element:**
- Silicon (Si):
![\[ \text{moles of Si} = \frac{10.85 \, \text{g}}{28.09 \, \text{g/mol}} \]](https://img.qammunity.org/2024/formulas/chemistry/college/n5inx49py4wghylhy3kkj1u470jkx8hbp4.png)
- Chlorine (Cl):
![\[ \text{moles of Cl} = \frac{27.40 \, \text{g}}{35.45 \, \text{g/mol}} \]](https://img.qammunity.org/2024/formulas/chemistry/college/zyc2odkdt5tdoksdk3viqe47mmk6afpxw4.png)
- Bromine (Br):
![\[ \text{moles of Br} = \frac{61.75 \, \text{g}}{79.90 \, \text{g/mol}} \]](https://img.qammunity.org/2024/formulas/chemistry/college/cibgs7mqjg6epggjypmq5l3r17cgydxuy0.png)
2. **Determine the mole ratios:**
- Divide each mole value by the smallest of the three.
- Round the ratios to the nearest whole number.
3. **Write the empirical formula:**
- The ratios obtained represent the subscripts of the elements in the empirical formula.
4. **Calculate the molar mass of the empirical formula:**
- Sum the molar masses of the elements in the empirical formula.
5. **Determine the molecular formula:**
- Use the given molar mass (258.8 g/mol) and the molar mass of the empirical formula.
Now, let's calculate:
![\[ \text{Moles of Si} = \frac{10.85 \, \text{g}}{28.09 \, \text{g/mol}} \approx 0.386 \]](https://img.qammunity.org/2024/formulas/chemistry/college/ko28d3k02nqp5myugi9hwf3k2hozk9zhjm.png)
![\[ \text{Moles of Cl} = \frac{27.40 \, \text{g}}{35.45 \, \text{g/mol}} \approx 0.772 \]](https://img.qammunity.org/2024/formulas/chemistry/college/9bil2tibu096smv2s6fmpmrx318dwyutrp.png)
![\[ \text{Moles of Br} = \frac{61.75 \, \text{g}}{79.90 \, \text{g/mol}} \approx 0.773 \]](https://img.qammunity.org/2024/formulas/chemistry/college/rea9rsy3c1zdzil6b15ozo9kzivxnlet88.png)
The mole ratios (rounded) are approximately:
![\[ \text{Si : Cl : Br} \approx 1 : 2 : 2 \]](https://img.qammunity.org/2024/formulas/chemistry/college/vk3wwuqautlz1bkvq3cyafy3s7raec0fjw.png)
The empirical formula is
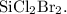
The molar mass of the empirical formula is calculated as follows:
![\[ (1 * \text{molar mass of Si}) + (2 * \text{molar mass of Cl}) + (2 * \text{molar mass of Br}) \]](https://img.qammunity.org/2024/formulas/chemistry/college/isy98h2txrpj1b148binh0xywuco23asq8.png)
![\[ = (1 * 28.09) + (2 * 35.45) + (2 * 79.90) \]](https://img.qammunity.org/2024/formulas/chemistry/college/lqst0rb185ixvd1s09j9fw8iupe887q18e.png)
![\[ = 28.09 + 70.90 + 159.80 \]](https://img.qammunity.org/2024/formulas/chemistry/college/55pyycaurdx3kjh94q5sh4t1uvr19o016q.png)
![\[ = 258.79 \, \text{g/mol} \]](https://img.qammunity.org/2024/formulas/chemistry/college/yzeyk7845er0cqk8ob0la75bqhrk2i9jb8.png)
Since the molar mass of the empirical formula matches the given molar mass (258.8 g/mol), the molecular formula is the same as the empirical formula:
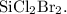
The probable question may be:
A compound is found to contain 10.85 % silicon , 27.40 % chlorine , and 61.75 % bromine by mass. To answer the question, enter the elements in the order presented above. QUESTION 1: The empirical formula for this compound is ____? QUESTION 2: The molar mass for this compound is 258.8 g/mol. The molecular formula for this compound is ___?