The rate law expression for the reaction is Rate = k[MnO4-]. The calculated rate constant (k) is
 To achieve a desired rate of
To achieve a desired rate of
 M/min with [MnO4-] of 0.040 M and [H+] of 0.100 M, [Fe2+] needs to be 0.160 M.
M/min with [MnO4-] of 0.040 M and [H+] of 0.100 M, [Fe2+] needs to be 0.160 M.
To determine the rate law expression for the reaction of iron (II) with permanganate in acidic solution, we analyze the given data.
Comparing Trial 1 and Trial 2, the concentration of H+ is doubled while the concentrations of Fe2+ and MnO4- remain the same, but the rate does not change significantly.
Therefore, H+ does not appear to affect the rate, suggesting zero order with respect to H+ ions.
Comparing Trial 1 and Trial 3, doubling the concentration of MnO4- doubles the rate, indicating first-order with respect to MnO4-.
From Trial 1 and Trial 4, increasing the concentration of Fe2+ by a factor of 3 changes the rate insignificantly, suggesting it is also not affecting the rate, or zero order with respect to Fe2+ ions.
Therefore, the rate law is Rate = k[MnO4-].
To calculate the rate constant (k), we use the data from Trial 1 where the rate is
 M/min and the concentration of MnO4- is 0.025 M.
M/min and the concentration of MnO4- is 0.025 M.
Substituting into the rate law gives k = rate/[MnO4-] = (
 M/min) / 0.025 M, which results in k =
M/min) / 0.025 M, which results in k =
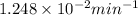
For question (c), we have the rate law and k, and we need the concentration of Fe2+ that would give a rate of
 M/min
M/min
The equation would be
 M/min =
M/min =
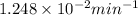 [Fe2+], solving for [Fe2+] gives [Fe2+] = 0.160 M.
[Fe2+], solving for [Fe2+] gives [Fe2+] = 0.160 M.
The probable question may be:
Use the data of the balanced chemical reaction, between iron (II) and permanganate in acidic solution, to answer the following questions (a-c):
5 Fe^{2+} (aq) + MnO_4^- (aq) + 8 H^+ (aq) → 5 Fe^{3+} (aq) + Mn^{2+} (aq) + 4 H_2O (l)
Trial [Fe^{2+}] [MnO_4^-] [H^+] rate
1 0.010 M 0.025 M 0.050 M 3.12x10^{-4} M/min
2 0.010 M 0.025 M 0.100 M 3.04x10^{-4} M/min
3 0.010 M 0.050 M 0.050 M 6.31x10^{-4} M/min
4 0.030 M 0.025 M 0.100 M 2.71x10^{-4} M/min
a. Determine the rate law expression of the reaction
b. Calculate k
c. What [Fe2+] is required to achieve a rate of 2.0 x 10-3 M/min when [MnO4] = 0.040 M and [H+] = 0.100 M?