Final Answer:
The question involves a classical physics scenario, and it's important to note that the concept of wavelength is typically associated with quantum mechanics and wave-particle duality. The given scenario doesn't directly align with the concept of wavelength for a ball. Therefore, it's not applicable to calculate the wavelength in this context.
Step-by-step explanation:
The wavelength of an object, especially a macroscopic one like a ball, is not a well-defined concept in classical physics. Wavelength is a property associated with waves and particles at the quantum level, as described by the de Broglie wavelength equation. In the context of everyday objects, such as a ball, we don't typically consider them to exhibit wave-like behavior.
If the question were related to a particle at the quantum level, we could use the de Broglie wavelength equation, which is given by:
![\[ \lambda = (h)/(p) \]](https://img.qammunity.org/2024/formulas/physics/high-school/1wyud5n6hma1h4jqu670dl5c01cd5qxxuk.png)
where
 i s the wavelength,
i s the wavelength,
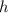 is the Planck constant, and
is the Planck constant, and
 is the momentum of the particle. However, for a macroscopic object like a ball, this equation isn't practically applicable.
is the momentum of the particle. However, for a macroscopic object like a ball, this equation isn't practically applicable.
In summary, the concept of wavelength, as described in the question, doesn't apply to a classical object like a ball moving at a certain velocity. It's crucial to recognize the appropriate context for physical concepts to ensure accurate and meaningful calculations.