Answer:
0.450 grams of Fe contains 4.85*10²¹ atoms
Step-by-step explanation:
Molar mass is the mass of one mole of a substance, which can be an element or a compound.
In this case, the molar mass of iron Fe is 55.85 g / mole. Then you can apply the following rule of three: if by definition of molar mass 1 mole of Fe contains 55.85 grams, then how many moles will contain 0.450 grams?
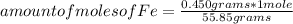
amount of moles of Fe=8.06*10⁻³ moles
On the other hand, Avogadro's Number is called the number of particles that make up a substance (usually atoms or molecules) and that can be found in the amount of one mole of said substance. Its value is 6.023*10²³ particles per mole. Avogadro's number applies to any substance.
Then you can apply the following rule of three: if by definition of Avogadro's Number 1 mole of Fe contains 6.023*10²³ atoms, 8.06*10⁻³ moles how many atoms does it contain?
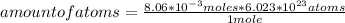
amount of atoms= 4.85*10²¹ atoms
0.450 grams of Fe contains 4.85*10²¹ atoms