Answer:
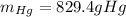
Step-by-step explanation:
Hello there!
In this case, considering the Avogadro's number, which helps us to realize that 1 mole of mercury atoms contains 6.022x10²³ atoms and at the same time 1 mole of mercury weights 200.59 g, we obtain:
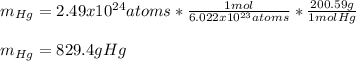
Best regards!