Answer:
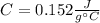
Step-by-step explanation:
Hello!
In this case, when doing calorimetry problems, we need to use the following equation:
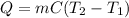
Whereas Q is the involved heat, m the mass, C the specific heat and T2 and T1 the final and initial temperatures respectively. Thus, since we need the specific heat, we proceed as follows:
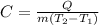
Thus, we plug in to obtain:
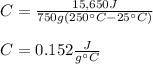
Best regards!