Answer:
The amount of potassium chloride that can dissolve in water increases by 0.3 grams for every 1 degree Celsius increase in temperature.
Step-by-step explanation:
Given
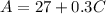
Required
Interpret 0.3
We make use of the following illustration to answer this question.
Let C = 1
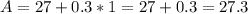
Let C =2
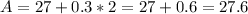
Let C = 3
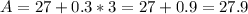
Notice that when the values of C increases by 1(i.e. from 1 to 2 and 2 to 3), the values of A increases by 0.3
i.e.
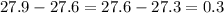
Hence, option D is true