Answer:
a) The mean repeat unit molar mass for PEVA is 30.72 g/mol
b) degree of polymerization of the copolymer is 1300
Step-by-step explanation:
Given that;
the wt% of copolymer consist of 12.9 wt% of vinyl acetate and 87.1 wt% Ethylene.
Basis: 100 g of PEVA consist of 12.9 of vinyl acetate and 87.1g of Ethylene.
now we calculate the mole fraction of vinyl acetate Ethylene in the copolymer;
the molecular weights of vinyl acetate and ethylene are 86.09 g/mol and 28.05 g/mol respectively
so
moles of vinyl acetate = wt. of vinyl acetate / molecular weights of vinyl acetate
moles of vinyl acetate = 12.9 g / 86.09 g/mol
moles of vinyl acetate = 0.1498 mol
moles of Ethylene = wt. of Ethylene / molecular weights of Ethylene
moles of Ethylene = 87.1 g / 28.05 d/mol
moles of Ethylene = 3.1052 mol
Total moles = 0.1498 mol + 3.1052 mol = 3.255 mol
Next we calculate the mole percent;
mole percent of vinyl acetate
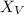 = moles of vinyl acetate / total moles
= moles of vinyl acetate / total moles
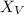 = (0.1498 mol / 3.255 mol) × 100
= (0.1498 mol / 3.255 mol) × 100
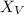 = 4.6%
= 4.6%
mole percent of Ethylene
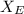 = moles of Ethylene / total moles
= moles of Ethylene / total moles
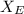 = (3.1052 mol / 3.255 mol) × 100
= (3.1052 mol / 3.255 mol) × 100
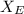 = 95.397% ≈ 95.4%
= 95.397% ≈ 95.4%
we know that, mean repeat unit molar mass for a sample = ∑
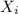
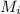
where
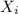 is the fraction ratio and
is the fraction ratio and
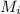 is the molecular weight
is the molecular weight
so or the PEVA
mean repeat unit molar mass M = (
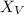
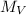 ) + (
) + (
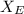
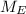 )
)
so we substitute
M = ( 4.6% × 86.09) + ( 95.4% × 28.05 )
M = 3.96014 + 26.7597
M = 30.72 g/mol
Therefore, The mean repeat unit molar mass for PEVA is 30.72 g/mol
b)
Degree of polymerization
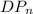 =
=
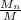
where
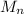 is the number average molecular weight ( 39,870 g/mol )
is the number average molecular weight ( 39,870 g/mol )
so we substitute
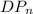 = 39,870 g/mol / 30.72 g/mol
= 39,870 g/mol / 30.72 g/mol
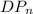 = 1297.85 ≈ 1300 { 3 significance figure }
= 1297.85 ≈ 1300 { 3 significance figure }
Therefore, degree of polymerization of the copolymer is 1300