Answer:
It will take 10.96 hours for 90% of the lead to decay.
Explanation:
The radioactive isotope of lead, Pb-209, decays at a rate proportional to the amount present at time t
This means that the amount can be modeled by the following function:
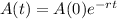
In which A(0) is the initial amount and r is the decay rate.
Has a half-life of 3.3 hours.
This means that
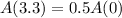 . We use this to find r.
. We use this to find r.
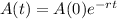
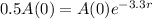
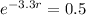
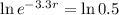
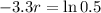
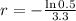
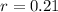
So
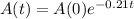
How long will it take for 90% of the lead to decay?
This is t for which
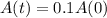 , that is, 100 - 90 = 10% of the initial amount.
, that is, 100 - 90 = 10% of the initial amount.
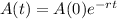
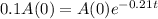
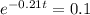
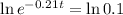
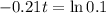
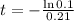
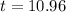
It will take 10.96 hours for 90% of the lead to decay.