The atomic radius of barium is approximately 0.222 nm.
Molar mass of barium: 137.33 g/mol
Avogadro's constant: 6.02214076 ×
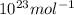
Density of barium: 3.51 g/cm³
First, convert the density to g/cm³:
density = 3.51 g/cm³
Then, calculate the volume per atom:
volume_per_atom = molar_mass / (density * Avogadro's constant)
volume_per_atom = 137.33 g/mol / (3.51 g/cm³ * 6.02214076 ×
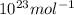 )
)
volume_per_atom = 5.85 ×
 cm³
cm³
Packing fraction: 0.68
The packing fraction represents the portion of the unit cell occupied by the atoms. We need to find the total volume of the unit cell to calculate the atomic radius.
volume_cell = volume_per_atom / packing_fraction
volume_cell = 5.85 ×
 cm³ / 0.68
cm³ / 0.68
volume_cell = 8.6 ×
 cm³
cm³
Since we know the unit cell is body-centered cubic, we can relate the volume to the edge length (a) using the formula for a cube:
volume_cell =
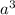
Taking the cube root of both sides:
a = (volume_cell)^(1/3)
a = (8.6 ×
 cm³)^(1/3)
cm³)^(1/3)
a = 0.413 nm
The atomic radius (r) is related to the edge length by the following equation:
r =
 a / 4
a / 4
Plugging in the value of a:
r =
 * 0.413 nm / 4
* 0.413 nm / 4
r = 0.222 nm