Final Answer:
The final temperature of the water after using a 200 W electric immersion heater can be determined using the formula
 , where
, where
 is the heat transferred,
is the heat transferred,
 is the mass of the water,
is the mass of the water,
 is the specific heat capacity of water, and
is the specific heat capacity of water, and
 is the change in temperature. In this scenario, assuming no heat is lost to the surroundings, the final temperature
is the change in temperature. In this scenario, assuming no heat is lost to the surroundings, the final temperature
 can be calculated to be approximately
can be calculated to be approximately
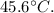
Step-by-step explanation:
To determine the final temperature of the water, we can use the formula
 , where:
, where:
 is the heat transferred (in joules),
is the heat transferred (in joules),
 is the mass of the water (in kg),
is the mass of the water (in kg),
 is the specific heat capacity of water
is the specific heat capacity of water
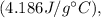
 is the change in temperature.
is the change in temperature.
The power
 of the electric immersion heater is given as 200 W. Since power is the rate of doing work, we can express it as
of the electric immersion heater is given as 200 W. Since power is the rate of doing work, we can express it as
 , where
, where
 is the time. In this case, if we consider the time to be 60 minutes (1 hour) for simplicity, we can find
is the time. In this case, if we consider the time to be 60 minutes (1 hour) for simplicity, we can find
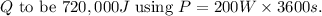
Now, substituting
 into the heat transfer formula, we get
into the heat transfer formula, we get
 Given that
Given that
 , rearranging the formula to solve for
, rearranging the formula to solve for
 Therefore, the final temperature of the water after the all-night study session is approximately
Therefore, the final temperature of the water after the all-night study session is approximately
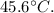
The complete question is:
"In an effort to stay awake for an all-night study session, a student makes a cup of coffee by first placing a 200 W electric immersion heater in 0.250 kg of water. If the initial temperature of the water is
 , what will be the final temperature of the water after using the immersion heater for one hour? Assume no heat is lost to the surroundings and use the specific heat capacity of water as
, what will be the final temperature of the water after using the immersion heater for one hour? Assume no heat is lost to the surroundings and use the specific heat capacity of water as
 ."
."