17.79 g
The number of grams of RbOH present in the solution can be found by using the formula
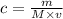
where
m is the mass in grams
M is the molar mass in g/mol
v is the volume in L or dm³
making m the subject we have
m = c × M × V
First of all we have to find the molar mass of RbOH
M(RbOH) = 85.5 + 16 + 1 = 102.5 g/mol
v = 31 ml = 0.031 dm³ or 0.031 L
c = 5.6 M
Substituting it into the formula we have
m = 5.6 × 102.5 × 0.031 = 17.79 g
We have the final answer as
17.79 g
Hope this helps you