Answer:
a) The difference in mercury levels in the manometer is 2 centimeters.
b) The gage of the gas is 2.670 kilopascals.
Step-by-step explanation:
a) Pressure in gases is absolute. A manometer helps to determine the hydrostatic difference between pressure of the gas (
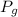 ) and atmospheric pressure (
) and atmospheric pressure (
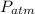 ), both measured in pascals. A kilopascal equals 1000 pascals and 1 meter equals 100 centimeters. That is:
), both measured in pascals. A kilopascal equals 1000 pascals and 1 meter equals 100 centimeters. That is:
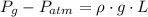 (1)
(1)
Where:
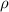 - Density of mercury, measured in kilograms per cubic meter.
- Density of mercury, measured in kilograms per cubic meter.
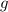 - Gravitational acceleration, measured in meters per square second.
- Gravitational acceleration, measured in meters per square second.
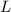 - Difference in mercury levels, measured in meters.
- Difference in mercury levels, measured in meters.
If we know that
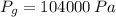 ,
,
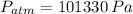 ,
,
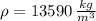 and
and
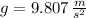 , the difference in mercury levels in the manometer is:
, the difference in mercury levels in the manometer is:
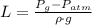
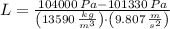
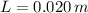
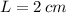
The difference in mercury levels in the manometer is 2 centimeters.
b) The gage pressure is the difference between gas pressure and atmospheric pressure: (
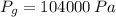 ,
,
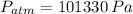 )
)
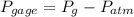 (2)
(2)
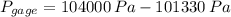
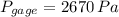
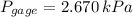
The gage of the gas is 2.670 kilopascals.