Answer: a)
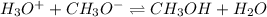
acid : hydronium ion
base : methoxide ion
conjugate acid : methanol
conjugate base: water
b)
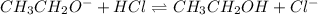
acid : hydrogen chloride
base : ethoxide ion
conjugate acid : ethanol
conjugate base: chloride ion
c)
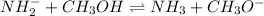
acid : methanol
base : amide ion
conjugate acid : ammonia
conjugate base: methoxide ion
Step-by-step explanation:
According to the Bronsted-Lowry conjugate acid-base theory, an acid is defined as a substance which looses donates protons and thus forming conjugate base and a base is defined as a substance which accepts protons and thus forming conjugate acid.
The species accepting a proton is considered as a base and after accepting a proton, it forms a conjugate acid.
The species losing a proton is considered as an acid and after loosing a proton, it forms a conjugate base
For the given chemical equation:
a)
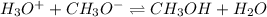
acid : hydronium ion
base : methoxide ion
conjugate acid : methanol
conjugate base: water
b)
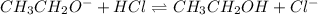
acid : hydrogen chloride
base : ethoxide ion
conjugate acid : ethanol
conjugate base: chloride ion
c)
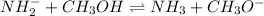
acid : methanol
base : amide ion
conjugate acid : ammonia
conjugate base: methoxide ion
.