Answer:
.0466 L
Step-by-step explanation:
To start off, use dimensional analysis to convert everything to standard PV=nRT units.
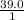 x
x
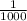 = .039 L
= .039 L
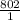 x
x
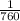 = 1.055 atm
= 1.055 atm
17 +273 = 290
...and so on.
Solve for n for the starting pressure, volume and temp. You'll need it for the final equation.
n= PV/RT
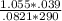 = .0017 number of moles
= .0017 number of moles
Solve for volume of the end point.
V= nRT/P
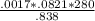 = .0466 L of helium, or 46.6ml Less pressure means it will take up more space.
= .0466 L of helium, or 46.6ml Less pressure means it will take up more space.