Answer:
See explanation.
Step-by-step explanation:
Hello!
In this case, these three equations show how the molecule behaves in terms aqueous and solid species; thus, we first write balanced conventional equation:
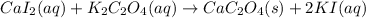
Whereas the solid product is CaC₂O₄ based off its low solubility. Next, we ionize the aqueous species to obtain the total ionic equation:
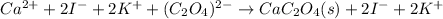
Finally, we cancel out potassium and iodide ions as they are the spectator ions due to their presence at both reactants and products in order to obtain the net ionic equation:
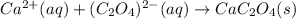
Best regards!