Answer:
455.6 kJ.
Step-by-step explanation:
Hello there!
In this case, according to the given reaction, we know that 2 moles of NO require 43 kcal of energy, thus, for the energy required by 150 g of NO we first need the moles, considering its molar mass (30.01 g/mol):
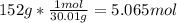
Thus, we apply the following dimensional analysis to obtain the energy absorbed by 5.065 moles:
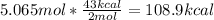
Which kJ turns out:
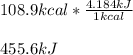
Best regards!