Answer:
D: 0.6 moles
Step-by-step explanation:
First we balance the equation:
- Zn + AgNO₃ → Zn(NO₃)₂ + Ag
There are two NO₃ species on the right side, so we put a two before AgNO₃:
- Zn + 2AgNO₃ → Zn(NO₃)₂ + Ag
Now we put a two before Ag on the right, to balance the Ag atoms:
- Zn + 2AgNO₃ → Zn(NO₃)₂ + 2Ag
The equation is now balanced.
Now we convert 3.6x10²³ atoms of zinc to moles, using Avogadro's number:
- 3.6x10²³ atoms ÷ 6.023x10²³ atoms/mol = 0.6 moles
Then we convert 0.6 moles of zinc into moles of silver, using the stoichiometric coefficients of the balanced equation:
- 0.6 mol Zn *
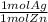 = 0.6 mol Ag
= 0.6 mol Ag
Thus the answer is option D.