Answer:
35.68g CO2
Step-by-step explanation:
we use the combustion equation with CH4:
CH4+ O2= CO2 + H2O
And then balance it:
CH4+ 2O2= CO2 + 2H2O
Using this equation we can use sociometry:
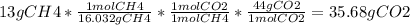
We know that 16.032 is how many grams there are in one mole of CH4 by adding the weights of the atoms (12 +1.008+1.008+1.008+1.008). These weights can be found on the periodic table. The same goes for the amount of grams per CO2.
The important thing about sociometry is to make sure your units cancel out until you are only left with the unit you want. If grams of CH4 is in the numerator, the next fraction you multiply by should have grams of Ch4 in the denominator. If moles of CO2 are in the numerator, the next fraction should have moles of CO2 in the denominator.