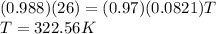Answer: 322.56 Kelvin
Step-by-step explanation:
Use the Ideal Gas Law

R is the gas constant
T is the temperature in Kelvins
P is the pressure in atmospheres
V is the volume in liters
n is the number of moles of gas
First, the mm of mercury need to be converted to atmospheres using the conversion factor 1atm = 760 torr.
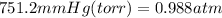
Now plug everything in