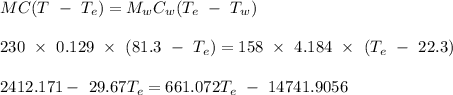The equilibrium temperature of the system, given that lead at 81.3 °C is placed in a calorimeter containing water at 22.3 °C, is 24.8 °C
How to calculate the equilibrium temperature of the sysytem?
The equilibrium temperature of the system can calculated by applying the conservation of thermal energy. This is shown below:
- Mass of lead (M) = 230 g
- Temperature of lead (T) = 81.3 °C
- Specific heat capacity of lead (C) = 0.129 J/g°C
- Mass of water (Mᵥᵥ) = 158 g
- Temperature of water (Tᵥᵥ) = 22.3 °C
- Specific heat capacity of water (Cᵥᵥ) = 4.184 J/g°C
- Equilibrium temperature of system (Tₑ) =?
Heat released by lead = Head gained by water