Answer:
V = 15.2 L
Step-by-step explanation:
STP means that T = 273 K and P = 1 atm.
We use the PV=nRT equation to convert the given liters of oxygen to moles:
- 1 atm * 7.6 L = n * 0.082 atm·L·mol⁻¹·K⁻¹ * 273 K
Now we convert O₂ moles to H₂O moles, using the stoichiometric coefficients of the equation:
- 0.340 mol O₂ *
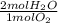 = 0.68 mol H₂O
= 0.68 mol H₂O
Finally we use the PV=nRT equation once again to convert 0.68 moles of H₂O to liters:
- 1 atm * V = 0.68 * 0.082 atm·L·mol⁻¹·K⁻¹ * 273 K