Final answer:
In the equation
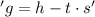 , 's' stands for the entropy change in the system, which is a measure of disorder or randomness.
, 's' stands for the entropy change in the system, which is a measure of disorder or randomness.
Step-by-step explanation:
In the equation
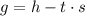 referred to in your question, 's' represents the entropy of the system. The term 'g' is the free energy change (ΔG), 'h' is enthalpy change (ΔH), and 't' is the absolute temperature in kelvin (T). The equation shows that the free energy change of a system is equal to the change in enthalpy minus the product of temperature and the change in entropy. The term ΔS is the entropy change, which is calculated as the sum of the entropies of the products minus the sum of the entropies of the reactants. It is important to account for the stoichiometric coefficients of the reactants and products when calculating the entropy change.
referred to in your question, 's' represents the entropy of the system. The term 'g' is the free energy change (ΔG), 'h' is enthalpy change (ΔH), and 't' is the absolute temperature in kelvin (T). The equation shows that the free energy change of a system is equal to the change in enthalpy minus the product of temperature and the change in entropy. The term ΔS is the entropy change, which is calculated as the sum of the entropies of the products minus the sum of the entropies of the reactants. It is important to account for the stoichiometric coefficients of the reactants and products when calculating the entropy change.