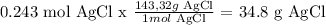Answer
the limiting reagent is NaCl
The mass of AgCl to be produced = 34.8 g
Step-by-step explanation
Given:
Balanced chemical equation: 1AgNO3+1NaCl=1AgCl+1NaNO3
mass of AgCl = ?
AgNO3 = 53.42 g
NaCl = 14.19 g
Solution
Step 1: Calculate the number of moles of reactants, to find the limiting reactant/reagent.
For AgNO3:
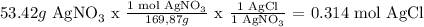
For NaCl
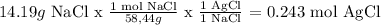
Therefore the limiting reagent is NaCl
Step 2: Calculate the mass of AgCl