Given:
The energy difference, E=2.4 eV
To find:
The wavelength of the emitted line.
Step-by-step explanation:
The energy of the wave emitted will be equal to the energy difference between the two energy levels.
The energy of the wave is given by the equation,
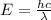
Where h is the Plank's constant and c is the speed of light.
The energy in joules is given by,
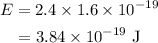
On substituting the known values in the equation of the energy,
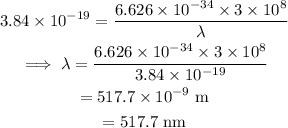
Final answer:
Thus the wavelength of the line is 517.7 nm